An 18.0 g sample of unidentified metal was heated from 21.5°C to 89.0°C.
292 J of heat energy was absorbed by the metal in the heating process.
Calculate the specific heat of the metal to determine its identity.(need work show, or how you got the answer)
A.calcium
B.iron
C.copper
D.silver
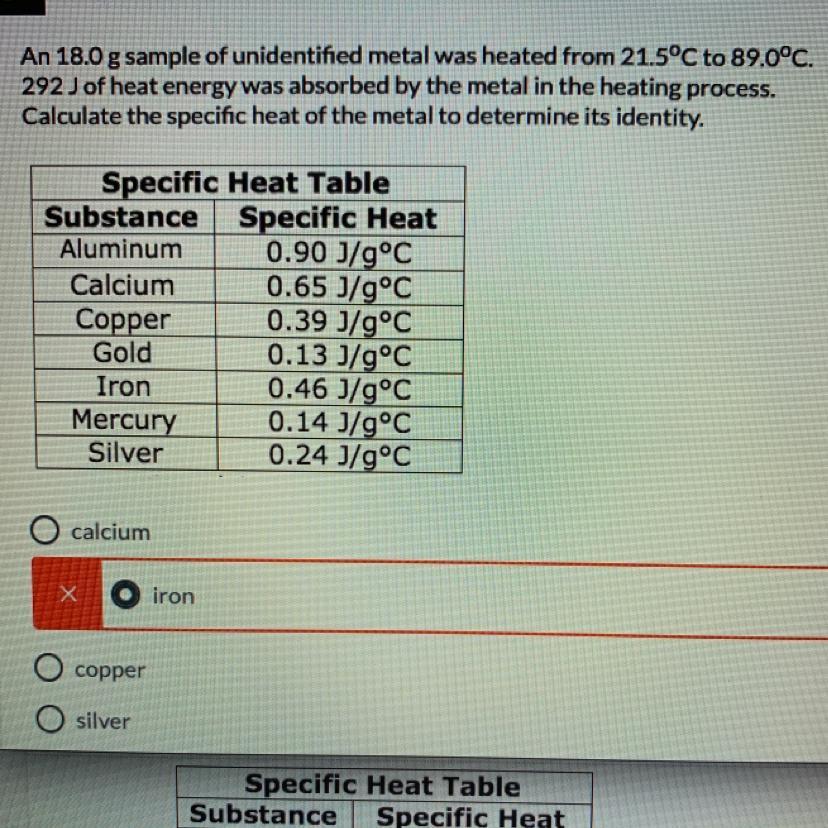
Answers
Answer: (d)-Silver
Explanation:
Given
The mass of sample is [tex]m=18\ gm[/tex]
It is heated from [tex]21.5^{\circ}C[/tex] to [tex]89^{\circ}C[/tex]
heat energy absorbed is [tex]292\ J[/tex]
Heat absorbed is given by [tex]Q=mc\Delta T[/tex]
Insert the values
[tex]\Rightarrow 292=18\times c\times [89-21.5]\\\Rightarrow 292=18\times c\times 67.5\\\\\Rightarrow c=\dfrac{292}{1215}\\\\\Rightarrow c=0.24\ J/g^{\circ}C[/tex]
As per the table, it is Silver
Related Questions
2 Iron(III) oxide (Fe2O3) is reduced by carbon on heating to give iron metal (Fe) and
carbon dioxide (CO2). (Ar values Fe 56, C 12,0 16)
When 480 g of Fe2O3 is heated with carbon, 336 g of Fe and 198 g of CO2 are produced.
Use the Law of Conservation of Mass to work out the mass of carbon that
reacted.
a
no links please i need this ASAP
Answers
Using the law of conservation of mass, the mass of carbon reacted is 54 g of carbon.
What is the law of conservation of mass?According to the law of conservation of mass, matter is neither created nor destroyed. This means that the total mass of reactants is equal to the total mass of products.
Total mass of the products = 336g + 198g = 534 g
Total mass of reactants = 480 g + x g
From the law of conservation of mass; 534 g = 480 g + x g
x g = 534 g - 480 g
x g = 54 g
Hence, 54 g of carbon reacted.
Learn more about law of conservation of mass: https://brainly.com/question/13383562
True or False: Molecular formula gives you the smallest whole number ratio of elements in a compound. *
Answers
Answer:true
Explanation:The molecular formula indicates the exact number of atoms in the molecule. The empirical formula expresses the smallest whole number ratio of the atoms in the element.
HELP ASAP
In which reaction are the atoms of elements rearranged?
Answers
Explanation:
A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does.
In a chemical reaction, the atoms and molecules that interact with each other are called reactants.
In a chemical reaction, the atoms and molecules produced by the reaction are called products.
In a chemical reaction, only the atoms present in the reactants can end up in the products. No new atoms are created, and no atoms are destroyed.
In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Answer:
It is B
Explanation:
The formation of water is a chemical reaction which rearranges atoms. The other reactions are nuclear reactions.
A salt is not composed of:
A. a metallic cation
B. non-metallic anion
C. an anion of a base
D. an anion of an acid
Answers
Answer:
B: non metalic anion if you try chemistry up salt is non metalic with metal things...
Explanation:
Does this help?
A heterogeneous mixture always contains
only one substance.
O more than two substances.
O two or more substances that are visibly distinguishable.
O two or more substances that are not visibly distinguishable.
Answers
Answer:
two or more substances that are visibly distinguishable.
Type the correct answer in each box.
Answers
Answer:
1 . 26
2. 55.854
Explanation:
because atomic number is the number of proton in other words smaller number and the mass number is the number of neutrons and electrons in other words bigger number .
hope this make sense :)
5 difference between Ionic compound and covalent compound
Answers
Answer:
Ionic compounds are formed by the transfer of electrons that are positively and negatively charged, whereas, covalent compounds are formed by sharing the electrons. 2. In an ionic compound, bonding involves a metal and nonmetal, whereas, in the covalent compound, bonding is between nonmetals
On the left a Circuit A, which is a square box. The top, left, and right sides have circles with X's in them. The bottom side has a stack of vertical lines, which are from left to right very short, short, very short, short. On the right a Circuit B, which is a square box. 2 extra lines cross the box near its top, parallel with the top side. The top side and the 2 extra lines have circles with X's in them. The bottom side has a stack of vertical lines, which are from left to right very short, short, very short, short.
Use the diagram and drop-down menus to answer each question.
Which circuit is a parallel circuit?
Which circuit is a series circuit?
In which circuit do the light bulbs all shine at their maximum brightness?
Answers
Answer:
B A B sorry if its wrong
Explanation:
Circuits can be connected in series or parallel but the maximum voltage is obtained in series connection, thus, light bulbs shine brightest in series connection.
What is parallel and series connection in a circuit?A parallel circuit connection is one in which the terminals of all the components in th circuit meet at two points.
The components are parallel to each other and current flows in multiple directions.
A series connection in a circuit is one in which the terminals of the component parts in a circuit are joined end to end. Current flows in one direction.
The voltage in a series connection is the sum of all the individual cells, therefore, the light bulb in series connection shines brightest.
Learn more about parallel and series circuits at: https://brainly.com/question/1122566
#SPJ2
Birds use their feathers for flight and fluff their feathers to keep warm. Based on Natalia's feather experiment, how do oil spills affect birds’ ability to use their feathers?
Answers
Answer:
As shown previously on Natalia's experiment, oil spills will dangerously affect birds' abilities to user their feathers. When oil gets on a feather, the mass of it increases and it becomes soggy and wet. If birds try to go in water like the ocean, it will not get any better because the ocean is cold. Flying won't be easy because they have additional weight stuck to them.
Explanation:
Hope this helps you!
How many moles of NO are made from
mixing 7.2 moles of NH3 and 9.6 moles
of O? Identify the limiting reagent.
Answers
Answer:
216 g of NO
Explanation:
We begin from the reaction:
4NH₃ + 5O₂ → 4NO + 6H₂O
We determine the limiting reactant with the moles of each reactant:
4 moles of ammonia react to 5 moles of oxygen
Our 7.2 moles of ammonia may react to (7.2 . 5) /4 = 9 moles
It's ok because we have 9.6 moles of oxygen. 0.6 moles still remain.
5 moles of oxygen react to 4 moles of NH₃
Our 9.6 moles of oxygen may react to (9.6 . 4) /5 = 7.68 moles
We only have 7.2 moles of NH₃ and we need 7.68; so there is no enough ammonia and that's our limiting reagent.
Now we determine the moles of product.
4 moles of ammonia can produce 4 moles of NO
Definetely our 7.2 moles, will produce 7.2 moles of oxide.
We convert to mass: 7.2 mol . 30 g/mol = 216 g
what do electrons in the same shell have in common
Answers
Answer:
They have the same amount of energy. They are all positively charged. They are all made up of atoms.
Answer:
they have the same quantity of energy...
If I have 6 moles of a gas at a pressure of 10.6 atm and a volume of 48 liters, what is the temperature of this gas?
An unknown quantity of gas is at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of 97 0C, how many moles of gas exist in this situation?
Answers
Answer: 1). The temperature of this gas is 1032.88 K.
2). There are 21.48 moles of gas exist at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of [tex]97^{o}C[/tex].
Explanation:
1). Given: No. of moles = 6 moles
Pressure = 10.6 atm
Volume = 48 L
Formula used to calculate temperature is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 atm
T = temperature
Substitute the values into above formula as follows.
[tex]PV = nRT\\10.6 atm \times 48 L = 6 mol \times 0.0821 L atm/mol K \times T\\T = \frac{10.6 atm \times 48 L}{6 mol \times 0.0821 L atm/mol K}\\= \frac{508.8}{0.4926} K\\= 1032.88 K[/tex]
Hence, temperature of this gas is 1032.88 K.
2). Given: Pressure = 14.5 atm
Volume = 45 L
Temperature = [tex]97^{o}C = (97 + 273) K = 370 K[/tex]
Formula used to calculate number of moles is as follows.
[tex]PV = nRT\\14.5 atm \times 45 L = n \times 0.0821 L atm/mol K \times 370 K\\n = \frac{14.5 atm \times 45 L}{0.0821 L atm/mol K \times 370 K}\\= \frac{652.5}{30.377} mol\\= 21.48 mol[/tex]
Hence, there are 21.48 moles of gas exist at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of [tex]97^{o}C[/tex].
what is electromagnetic?
Answers
Answer:
relating to the interrelation of electric currents or fields and magnetic fields.
Helpp me please answer fast
Answers
Answer: An element is a substance only containing atoms that have the same number of protons in the chemical nuclei
Explanation:
What mass of potassium would need 2350 J of energy in order to raise its temperature from 44.3°C to 57.8°C? (cpotassium = 0.753 J/g°C)
Answers
Answer:
Explanation:
q = mCΔT
m =CΔT/q
ΔT = 57.8°C - 44.3°C = 13.5°C
m = (0.753*13.5)/2350
m = 0.00433 g
The mass of potassium that will need 2350 J of energy in order to raise its temperature from 44.3°C to 57.8°C is 231.17 g
Data obtained from the question Heat (Q) = 2350 JInitial temperature (T₁) = 44.3 °CFinal temperature of water (T₂) = 57.8 °CChange in temperature (ΔT) = 57.8 – 44.3 = 13.5 °C Specific heat capacity (C) = 0.753 J/gºC Mass (M) =?Q = MCΔT
2350 = M × 0.753 × 13.5
2350 = M × 10.1655
Divide both side by 10.1655
M = 2350 / 10.1655
M = 231.17 g
Learn more about heat transfer:
https://brainly.com/question/6363778
#SPJ2
Describe and show an example of valence electrons. What is a common trend you can remember to help you understand valence electrons?
Answers
Explanation:
valency from right to left in a Periodic Table in same period first increases then decreases and from top to bottom it is same for a same group
what is chemical bonding?
Answers
Answer:
Chemical bonding - a mutual adhesion of atoms in an molecule and the crystal lattice.
Explanation:
Hope you have a great day
What is the percent by mass of carbon in carbon dioxide (CO2)?
A. 27%
B. 33%
C. 43%
D. 73%
Answers
What is the hydroxide ion concentration of a solution with a pH of 4?
A. 1x10^-4
B. 1x10^-7
C. 1x10^-14
D. 1x10^-10
Answers
Answer:
Jules
Explanation:
Help meeeeee
A 280.0 sample of neon gas exerts a pressure of 660.0 mm Hg at 26.0°C. Assuming that the pressure remains constant, at what temperature in would the volume change to 440.0 ml?
Answers
Answer:
469.9K
Explanation:
Using Charles's law equation:
V1/T1 = V2/T2
Where;
V1 = initial volume (ml)
V2 = final volume (ml)
T1 = initial temperature (K)
T2 = final temperature (K)
Based on the information provided:
V1 = 280.0ml
V2 = 440.0 ml
T1 = 26.0°C = 26 + 273 = 299K
T2 = ?
Using V1/T1 = V2/T2
280/299 = 440/T2
0.936 = 440/T2
T2 = 440 ÷ 0.936
T2 = 469.9K
The best way to increase the amount of a solid solute dissolved in a saturated solution would be to
Answers
Answer:
The solubility of a saturated solution can be increased by increasing the temperature.
Explanation:
Temperature -- Generally, an increase in the temperature of the solution increases the solubility of a solid solute. For example, a greater amount of sugar will dissolve in warm water than in cold water. A few solid solutes, however, are less soluble in warmer solutions.
How many pairs of electrons are shared by adjacent carbon atoms in an alkane?
Answers
Answer:
one
Explanation:
Help i’m taking a test!!
What new element is formed from the equation below? (Answer form is
MN:, AN: , S)
Answers
Answer:
Element Z
Explanation:
I hope this is correct and helps you!
Which of the following is not a fossil fuel?
o copper
natural gas
O petroleum
O coal
Answers
Answer:
copper
Explanation:
coal, petroleum, and natural gas are all related to fossil fuels.
Copper is not a fossil fuel and the correct option is option 1.
What are fossil fuels?Fossil fuels are compound mixtures made of fossilized plant and animal remnants from millions of years ago. The creation of fossil fuels—either oil, natural gas, or coal—from these fossils is determined by the type of fossil, the amount of heat, and the amount of pressure.
Fossil fuels are a non-renewable source of energy. Most of the energy used by us is obtained by the burning of fossil fuels. These fossil fuels are used up at a faster rate. They cannot be regrown at a scale compared to their consumption.
Therefore, Copper is not a fossil fuel and the correct option is option 1.
Learn more about Fossil fuels, here:
https://brainly.com/question/3371055
#SPJ2
Find the total pressure (in atm) of a mixture that contains 3 gases with the following partial pressures. Gas A is 4.2atm, Gas B is 780 mmHg, Gas C is 250.00kPa
Answers
Answer: The total pressure of given mixture that contains 3 gases is 7.68 atm.
Explanation:
Given: Partial pressure of gases is:
Gas A = 4.2 atm
Gas B = 780 mm Hg
Convert mm Hg into atm is as follows.
[tex]1 mm Hg = 0.00131579 atm\\780 mm Hg = 780 mm Hg \times \frac{0.00131579 atm}{1 mm Hg}\\= 1.02 atm[/tex]
Gas C = 250 kPa
Convert kPa into atm as follows.
[tex]1 kPa = 0.00986923 atm\\250 kPa = 250 kPa \times \frac{0.00986923 atm}{1 kPa}\\= 2.46 atm[/tex]
Total pressure is the sum of partial pressures of gases present in the mixture.
Therefore, total pressure of given mixture is calculated as follows.
[tex]P_{total} = P_{A} + P_{B} + P_{C}\\= 4.2 atm + 1.02 atm + 2.46 atm\\= 7.68 atm[/tex]
Thus, we can conclude that the total pressure of given mixture that contains 3 gases is 7.68 atm.
147.0 g piece of metal was heated to 155 C and added to a calorimeter containing 75.0 grams of water. The temperature of the water in the calorimeter was 23.5 C. After the metal was added, the temperature rose to 33.5 C. What is the specific heat of the metal?
Answers
Titaium oxside is often added to food to color it white. If a jelly bean contains approximately 1.28x10-5 moles of TiO2, how many grams of TiO2 are in a jelly bean
Answers
Answer:
1.02 × 10⁻³ g
Explanation:
Step 1: Given data
Number of moles of titanium (IV) oxide in 1 jelly bean (n): 1.28 × 10⁻⁵ moles
Step 2: Calculate the mass (in grams) corresponding to 1.28 × 10⁻⁵ moles of TiO₂
To convert moles to mass, we need a conversion factor. In this case, it is the molar mass of TiO₂: 79.87 g/mol.
1.28 × 10⁻⁵ mol TiO₂ × 79.87 g TiO₂/1 mol TiO₂ = 1.02 × 10⁻³ g TiO₂
Identify two ways that temperature plays a role in chemical changes.
Answers
Answer:
Increasing the temperature will cause chemical changes to occur faster. Decreasing the temperature, causes the particles to lose energy which causes them to move around less and slower. The less they move, the less collisions occur, and the less reactions occur between the chemicals = slower reaction rate.
Explanation:
Would an alkali metal make a good replacement for tin in a tin can? Explain.
Answers
Answer:
No
Explanation:
If tin is heated, it can react with alkalis' with the release of hydrogen.
Tin is a great metal that is used for packaging purposes.
It does not react with any acid that may be present in the food material stored inside the tin can which makes it a useful metal. It keeps the food safe.However, if the tin in the tin can is replaced with alkali metals then they will readily react with the acid present in the food materials stored inside, thereby rendering the food unsafe.
https://brainly.com/question/18153051
What will the volume be of 50 moles of helium at 25°C and 2.5 atm?